De wespen bouwen graag hun nesten in de openingen van instrumenten die worden gebruikt om de snelheid van vliegtuigen te meten. En dat is een groter probleem dan gedacht.
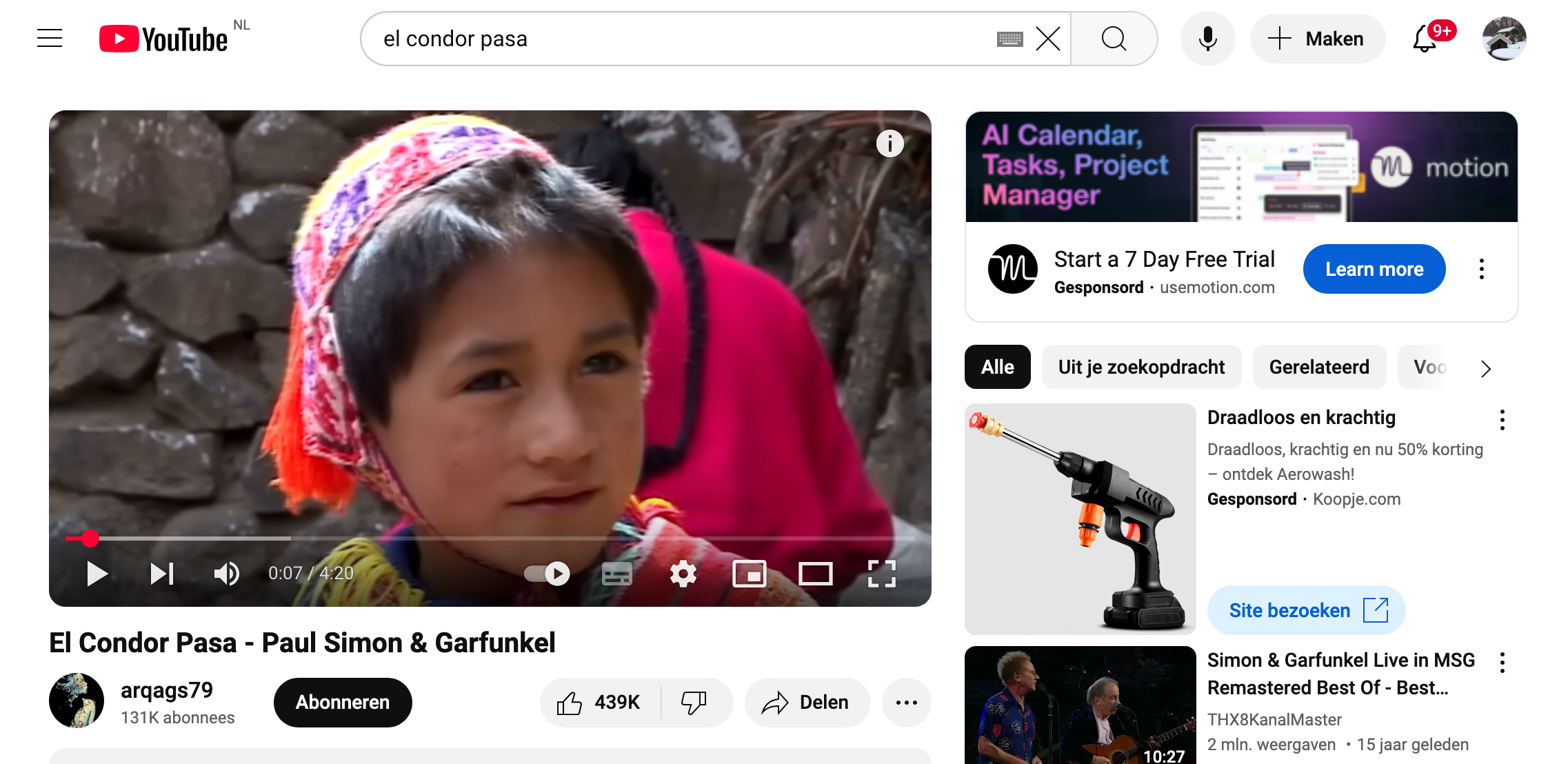
In Australië hebben ze last van een nogal vervelende wesp. Het gaat om de zogenoemde ‘sleutelgatwesp’, ofwel de Pachodynerus nasidens. De wespen bouwen graag hun nesten in kleine gaatjes, inclusief de openingen van instrumenten die worden gebruikt om de snelheid van vliegtuigen te meten. En dat leidt tot buitengewoon gevaarlijke situaties.

Meer over de Pachodynerus nasidens
De Pachodynerus nasidens komt oorspronkelijk voor in Zuid- en Midden-Amerika en het Caribisch gebied. Ondertussen komt hij echter ook op veel andere plekken voor, zoals bijvoorbeeld in Australië. Deze wesp staat erom bekend nesten te bouwen in door de mens gemaakte holtes. Denk bijvoorbeeld aan raamspleten, stopcontacten en – zoals zijn naam al doet vermoeden – sleutelgaten.
Ganzen
Aanvaringen tussen vliegtuigen en wilde dieren komen jammer genoeg veelvuldig voor. Denk bijvoorbeeld aan botsingen tussen vogels en vliegtuigen en dan in het bijzonder de Canadese gans. Deze reizen in troepen en zijn in die hoedanigheid in staat om de motoren van een groot passagiersvliegtuig uit te schakelen. Hierdoor zijn er in het verleden al hele vliegtuigen verwoest, mensen gewond geraakt en kwamen er zelfs passagiers om het leven.
Wesp
In het vorige voorbeeld hadden we het nog over een relatief groot dier dat in staat is om een vliegtuig te laten neerstorten. Maar opmerkelijk genoeg kunnen ook hele kleine insecten schade aanrichten. Tussen november 2013 en april 2019 werden er in totaal 26 gevallen gemeld van wespen die op luchthavens voor gevaarlijke situaties hadden gezorgd. Bovendien verstoppen de zogenoemde sleutelgatwespen met hun nesten de pitotbuizen; cruciale instrumenten die de snelheid van een gas- of vloeistofstroom meten. Deze buizen worden onder andere toegepast als snelheidsmeter in vliegtuigen.
In een nieuwe studie onderzochten wetenschappers de mogelijke rol van sleutelgatwespen bij het belemmeren van pitotbuizen op de luchthaven in de Australische stad Brisbane. De onderzoekers bouwden een aantal pitotbuizen na en monteerden deze vervolgens op vier verschillende locaties op het vliegveld. Al snel kwamen de wespen op de buizen af. De wespen leken met name een voorkeur te hebben voor de pitotbuizen waarin de temperatuur zo tussen de 24 en 31 graden Celsius lag en waarvan de opening een diameter had van zo’n 3 mm. Over een periode van 39 maanden wisten de invasieve wespen maar liefst 93 keer een nagemaakte pitotbuis volledig te blokkeren. “Deze resultaten onderstrepen het belang van risicobeperkende maatregelen,” zo merken de auteurs op.
Maatregelen
Volgens de onderzoekers vormen de wespen een groot risico voor de luchtvaartveiligheid. Ze bepleiten dan ook dat er maatregelen moeten worden genomen. Denk aan het afdekken van pitotbuizen wanneer vliegentuigen arriveren of het neerzetten van meer wespenvallen om zo gevaarlijke situaties te voorkomen. “We zouden nieuwe strategieën moeten ontwikkelen om deze aanpassende, inventieve en zeer mobiele soort te beheersen of zelfs uit te roeien,” zo claimen de onderzoekers.
Hoewel je het misschien niet zou verwachten van zo’n klein beestje, kunnen wespen dus een behoorlijk impact hebben op de luchtvaart. “We hopen dat dit onderzoek de aandacht vestigt op een weinig bekend, maar ernstig probleem voor de luchtvaart in tropische en subtropische gebieden,” zo concluderen de onderzoekers. “Sinds de wesp een weg over de Stille Oceaan heeft gevonden, is er geen reden om eraan te twijfelen dat hij zich ook naar andere delen van Australië zou kunnen verspreiden. Als we er niets aan doen om deze soort te beheersen, kunnen de gevolgen van deze plaag aanzienlijk worden.”

Introduction (Back to Top)

Pachodynerus erynnis (Lepeletier) (Figure 1) is a predatory wasp that is a specialist predator of caterpillars, the larvae of moths and butterflies (Lepidoptera). This insect does not yet have an officially accepted common name and has been referred to as the red and black mason wasp, red-marked Pachodynerus, and simply as a mason wasp. As with other closely related wasps, Pachodynerus erynnis, does not form a communal hive, but builds solitary nests in holes or crevices of trees, manmade structures, and abandoned nests created by other cavity-nesting bees and wasps. The red and black mason wasp frequently visits flowering plants and can be found entering nesting cavities near flowers. This insect is considered highly beneficial because it feeds on several key caterpillar pests, including armyworms (Spodoptera spp.), cutworms (Agrotis spp.), and loopers (Noctuoidea) (Krombein 1967), and has been associated with increased pest control in managed landscapes (Dale et al. 2019). Although fairly common, this wasp often goes unnoticed due to its solitary nature and quick flight.
Distribution (Back to Top)
Although most commonly reported from the southeastern United States, Pachodynerus erynnis is widely distributed from the coasts of Florida to North Carolina and ranges west to eastern Texas (Buck et al. 2008, iNaturalist). The species has rarely been sighted north of its usual range, with the few such sightings attributed to the wasp being transported by storms or human activities (Buck et al. 2008). Most of the 45 described species of Pachodynerus inhabit tropical regions of the New World (Bushini and Buss 2009). Only four other species occur in the southern United States, one of which, the keyhole wasp (Pachodynerus nasidens), is also found in Florida.
Description (Back to Top)

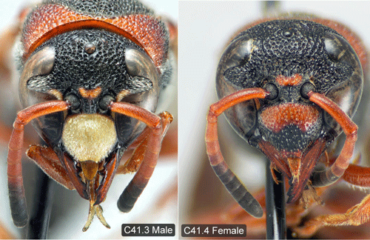
Pachodynerus erynnis is a relatively small wasp, with the front pair of wings (forewings) measuring just under 0.39 inches (1 cm) in males and only slightly longer in females (Buck et al. 2008). As the common name suggests, the adult wasps have both black and dull rusty-red coloration alternating around their bodies. The red and black coloration creates a red triangular shape when viewed from the side and a red horseshoe shape on the thorax, just behind the head, when viewed from above (Figure 1). As noted by Deyrup et al. (2003), this general color combination is commonly found in Florida wasps; however, Pachodynerus erynnis can easily be identified from other similarly colored wasps by its completely black coloration of the head behind the eyes. Other species have a red spot located behind the eyes. Interestingly, the male wasps have yellow markings on a select few regions of their head. The most distinct marking on the male’s head is the yellow clypeus, an almost hexagonal shield-like plate on the front of the insect’s face (Figure 2). On females, the clypeus is dull red and black like the rest of their body (Figure 2) (Buck et al. 2008).
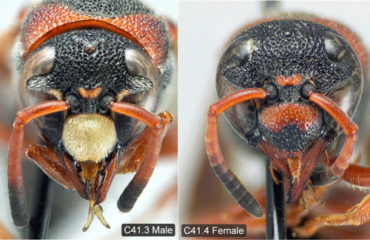
The purpose of the yellow markings on the male wasp head is unknown, but it has been hypothesized that they act as a form of visual communication (Deyrup et al. 2003). Pachodynerus erynnis males can furthermore be differentiated from females and other wasp species in the same family (Vespidae) because they have only 10 antennal segments (flagellomeres) instead of 11 antennal segments typical of females and other wasp species (Buck et al. 2008).
Building the Nest: Pachodynerus erynnis, like other species in its genus, are solitary wasps. This means that unlike some other communal wasps (e.g., paper wasps) or social bees, Pachodynerus females lay their eggs in individual nests, which are not tended to by any other individuals (Bushini and Buss 2009). Before the eggs can be deposited, the female wasp must find or build a suitable nest cavity. A true opportunist, this wasp will reuse uninhabited nests from other wasps. For example, in Florida, preferred nesting sites are old nests from the potter wasp, Zeta argillaceum (Matthews and Gonzales 2003). The red and black mason wasp has also been known to make nests within manmade structures around homes or buildings, including keyholes and wooden fence cavities (Krombein 1967). As long as the nesting site is a relatively straight, narrow tubular shape between half an inch to five inches long (1.27 cm to 12.7 cm), the wasp should be able to modify the tube to fit her needs (Krombein 1967).
A typical nest structure begins with an interior plug at the deepest point of the cavity comprised of aggregated sand and grit particles. She will then deposit one egg in this cell, and then provision the cell by placing paralyzed caterpillars into the cell for the wasp larva to consume. Next, she will make another plug, again out of aggregated sand and grit particles, to seal the cell and protect the developing larva inside (Figure 3) (Krombein 1967). The wasp will repeat the cell building process until she reaches the end of the cavity. The physical structure of sand plugs serves another important purpose for the developing insect. The side of the plug marking the direction of the exit is always convex and rough, while the side marking the direction of the interior of the nest is always smooth and concave (Figure 3), a shape resulting from the mother wasp smoothing out the plug with her rounded head. These structural and textural differences serve as a rudimentary compass for the emerging adults from within the nest after development, as the recently developed wasps know to chew the rough side (Krombein 1967).
Pachodynerus erynnis is interesting because instead of building only provisioned cells and partitions, they sometimes build an intercalary cell, which is simply an empty cell built in-between provisioned cells in random patterns (Krombein 1967). The biological reason for this is unknown. Nests may contain one to eight provisioned cells, but typically contain an average of four (Krombein 1967). The final, most exterior cell in the nest is called a vestibular cell, which is left empty. Lastly, the wasp will close off the entrance to the nest with a final plug of aggregated sand, which is quite durable and thick to keep parasitoids and other organisms out of the nest (Krombein 1967).
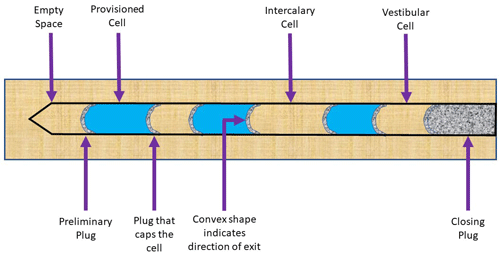
Figure 3. Depiction of a Pachodynerus erynnis (Lepeletier) nest, containing three provisioned cells, two intercalary cells, one vestibular cell, and sand/grit aggregate plugs sealing off the cells. Diagram is a modification of that presented in Krombein (1967) and was illustrated by Kelly Laplante, 2020.
Eggs: An adult female Pachodynerus erynnis lays a single egg into each cell in her nest. The egg is suspended from the ceiling by a thin stalk, providing a secure location for the egg to develop (Buck et al. 2008). Interestingly, the female wasp always lays female eggs towards the interior of the nest and male eggs towards the exterior of the nest. This way, males, which have a slightly shorter development time, can exit the nest before the females. Immediately before laying each egg, the female is able to control the future sex of her young by choosing to fertilize the egg or not. Fertilized eggs develop into females and unfertilized eggs develop into males (Buck et al. 2008). After the egg is laid, the cell will be provisioned and then closed off before the female begins building another cell and laying the next egg.
Larvae: After emerging from the egg, a Pachodynerus erynnis larva will consume the caterpillars that were paralyzed and left in the provisioned cell by the adult female. Seven to ten caterpillars can be found in each cell, with more provided for female wasp larva than male wasp larva (Buck et al. 2008). After feeding is complete and the wasp larva is plump and nearly circular in cross section, the larva will begin to pupate. Male wasp larvae take approximately seven days to begin pupation after feeding is complete, while a female will take seven to 10 days (Krombein 1967). During this time, the wasp larva will take on a flattened and dull appearance, expel any excess waste, and start spinning a cocoon (Krombein 1967).
Pupae: The cocoon, created from a delicate, semitransparent silk, serves as a protective shell encasing the pupa of Pachodynerus erynnis. Female cocoons are slightly larger than males, ranging from 0.47 to 0.67 inches (12 to 17 mm) long with an average of 0.54 inches (13.8 mm). Male cocoons range from 0.4 to0.67 inches (10 to 17 mm) long with an average of 0.46 inches (11.7 mm) (Krombein 1967). The length of time spent in the cocoon is variable, with males developing in around 18 to 28 days, and females 19 to 33 days (Krombein 1967).
Adult: After pupating and emerging from the cocoon, the now adult Pachodynerus erynnis will wait a day or two for its soft exoskeleton to harden, and then emerge from the nest (Krombein 1967). Individuals in the outermost cells will complete development and emerge from the nest prior to those in the innermost cells. Adult body length size ranges from 0.28 to 0.55 inches (7 to 14mm). As mentioned before, the adult wasp knows which direction to exit the nest based on the curvature and texture of the cell divider, chewing through the rougher, convex side. Once emerged, a female wasp will mate and then begin hunting and nest building (Figure 4). Pachodynerus erynnis has multiple broods and is active year-round, except for one to three of the coldest months of winter, during which time the wasp will overwinter (or go dormant) until the weather becomes warmer (Krombein 1967).
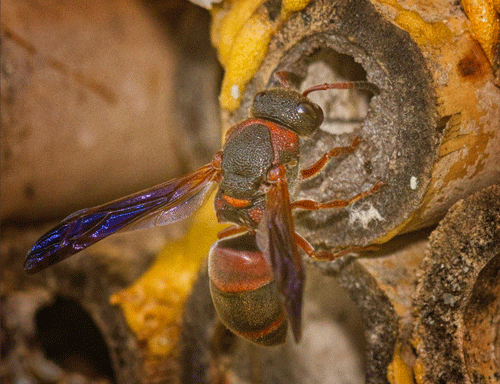
Figure 4. A female Pachodynerus erynnis (Lepeletier) working on a nest within a bamboo stalk. Photograph by Fitz O. Clarke Jr., 2015.
Hosts (Back to Top)
During the day, Pachodynerus erynnis females hunt and capture live caterpillars (moth larvae) and carry them to their nest. Captured prey are paralyzed with a sting and carried to nest cells to provide food for the wasp larvae once they hatch. This wasp has been observed attacking several prey species within at least seven families of caterpillars within the order Lepidoptera. Known prey includes species in the families: Erebidae, Crambidae, Oecophoridae, Amphisbatidae, Elachistidae, Coleophoridae, Noctuidae, and Tortricidae (Buck et al. 2008). The moth families mentioned include several economically important pests such as the light brown apple moth (Epiphyas postvittana), Nantucket pine tip moth (Rhyacionia frustrana), pecan bud moth (Gretchena bolliana), strawberry leafroller (Ancylis comptana), European corn borer (Ostrinia nubilalis), pickleworm (Diaphania nitidalis), pine webworm (Pococera robustella), fall armyworm (Spodoptera frugiperda), corn earworm (Helicoverpa zea), cabbage looper (Trichoplusia ni), and black cutworm (Agrotis ipsilon). In addition to providing their larvae with plenty of caterpillars to feed on, adults must also forage for themselves. Throughout the day adults can be seen visiting flowers of many different plant species, eating pollen and nectar.
Importance (Back to Top)
The economic value of Pachodynerus erynnis has not been quantified and would be very difficult to determine due to its elusive, solitary nature and presence across multiple habitat types; however, this insect does provide valuable pest control services that can reduce damaging pest populations in various plant systems. For example, Pachodynerus erynnis was the most abundant colonizer of pollinator nesting boxes placed in flowering habitats on golf courses in north-central Florida, where a 50% increase in predation of fall armyworm on maintained turfgrass was observed (Dale et al. 2019). Increasing red and black mason wasp abundance on golf courses and other vulnerable plant systems may translate to greater biological control of damaging plant pests, which can reduce pesticide use and consequent plant damage.
Due to the feeding behavior of this insect, it is an excellent candidate for conservation biological control efforts to increase its abundance and associated control of nearby pests. The most effective way to manage this insect is by providing nesting habitat and nutritional resources. The wasp utilizes flowering plants, such as the saw palmetto (Serenoa repens) or wildflowers, as sources of nectar and pollen (Koptur and Khorsand 2018). One study from north-central Florida in 2017 showed that planting mixtures of five and nine native wildflower species increased wasp abundance and biological control of fall armyworm caterpillars (Dale et al. 2019). Providing pollen-producing plants as a food source for the adult wasp may promote the establishment of this species and other beneficial insects in nearby habitats.
Perhaps the most important step to attracting this wasp is the addition of nesting boxes (Campbell et al. 2017). Pollinator nesting boxes with hollow reeds or wooden blocks with holes drilled into them, as seen in Figures 5, 6 and 7, have been shown to increase the local abundance of this species and other beneficial vespid wasps (Campbell et al. 2017). Such pollinator nesting boxes should be placed in areas near flowering plants to provide Pachodynerus erynnis with nesting habitat and floral resources, thereby locally increasing their biological control services and protecting economically or aesthetically important plants in the landscape from caterpillar pests. Importantly, nesting boxes do not need to be in or directly adjacent to flowering plants to provide value. Dale et al. (2019) found that nesting boxes simply placed in turfgrass areas were colonized at greater rates than those placed within wildflower habitats.
More information on building your own pollinator nesting box can be found here: https://secure.caes.uga.edu/extension/publications/files/pdf/C%201125_1.PDF . To specifically target Pachodynerus erynnis, nest holes should be drilled with diameters of 3/16 to 1/4 inches (4.8 mm to 6.4 mm) and to a depth ranging from half an inch to five inches (14 to 125 mm) to be consistent with naturally observed nest measurements (Krombein 1967).

Figure 5. A pollinator nesting box containing hollow bamboo reeds being visited by two Pachodynerus erynnis (Lepeletier) adults. Photograph by Fitz O. Clarke Jr., 2015.

Figure 6. A pollinator nesting box with cavities at various stages of Pachodynerus erynnis (Lepeletier) use, with an adult wasp emerging. Photograph by Fitz O. Clarke Jr., 2015.

Figure 7. Full view of a pollinator nesting box with cavities at various stages of Pachodynerus erynnis (Lepeletier) use. Photograph by Adam Dale, University of Florida.
Selected References (Back to Top)
- Buck M, Marshall SA, Cheung DKB. 2008. Identification atlas of the Vespidae (Hymenoptera, Aculeata) of the northeastern Nearctic region. Canadian Journal of Arthropod Identification 05. p. 492 . Available from: http://doi.org/10.3752/cjai.2008.05 (10 April 2020)
- Buschini MLT, Buss CE. 2009. Biological aspects of different species of Pachodynerus. Brazilian Journal of Biology 70(3): 623-629. Available from: https://doi.org/10.1590/S1519-69842010000300020[10 April 2020]
- Campbell JW, Smithers C, Irvin A, Kimmel CB, Stanley-Stahr C, Daniels JC, Ellis JD. 2017. Trap nesting wasps and bees in agriculture: A comparison of sown wildflower and fallow plots in Florida. Insects 8(4): 1-10 . Available from: https://doi.org/10.3390/insects8040107 (10 April 2020)
- Carpenter, JM. 1986. The Genus Pachodynerus in North America (Hymenoptera: Vespidae: Eumeninae). Proceedings of the Entomological Society of Washington, 1986. pp 572-577. Available from: https://www.biodiversitylibrary.org/item/54986#page/596/mode/1up (10 April 2020)
- Dale AG, Perry RL, Cope GC, Benda N. 2019. Floral abundance and richness drive beneficial arthropod conservation and biological control on golf courses. Urban Ecosystems 23(1): 55-66. Available from: https://doi.org/10.1007/s11252-019-00907-0 (10 April 2020)
- Deyrup M, Eisner T. 2003. Red and black coloration in Florida Hymenoptera. Southeastern Naturalist 2(4): 511-522. Available from: https://doi.org/10.1656/1528-7092(2003)002[0511:RABCIF]2.0.CO;2. [10 April 2020]
- Koptur S, Roxaneh K. 2018. Pollination ecology of three sympatric palms of southern Florida pine rocklands. Natural Areas Journal 38(1): 15-25. Available from: https://doi.org/10.3375/043.038.0104 (10 April 2020)
- Krombein KJ. 1967. Trap-nesting Wasps and Bees: Life histories, nests, and associates. Smithsonian Press. pp 570. Available from: https://library.si.edu/digital-library/book/trapnestingwasps00krom (10 April 2020)
- Linton E. 2006. Beneficial Insects in the Garden: #39 Red & Black Mason Wasp (Pachodynerus sp.). Galveston County Master Gardeners, Texas A&M University. Available from: https://aggie-horticulture.tamu.edu/galveston/beneficials/beneficial-39_red_and_black_mason_wasp.htm (10 April 2020)
- Matthews RW, Gonzalez JM. 2003. Nesting biology of Zeta argillaceum in southern Florida U.S. Florida Entomologist 87(1): 37-40. Available from: https://doi.org/10.1653/0015-4040(2004)087[0037:NBOZAH]2.0.CO;2 (10 April 2020)